Setting up a new Western blot? Thanks to increased assay sensitivities, readily available antibody-coupling products and improved conjugate reagents, you have a big decision to make: Should you do a direct or an indirect Western blot?
The technical difference between direct and indirect Westerns can be summed up easily – a direct Western blot uses one antibody for detection while an indirect Western uses two antibodies. While this explanation is simple, this slight change in protocol has meaningful consequences for your experiment.
Indirect detection
Traditionally, the most popular type of Western is an indirect Western. Using the indirect method, you first incubate your blot with a primary antibody that recognizes your protein of interest. You then incubate with a labeled secondary antibody that recognizes the primary antibody. This is illustrated in the picture below:
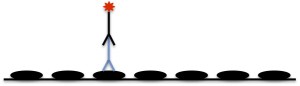
(If these pictures look familiar, thank you for reading our blog more than once! This same principle is applicable in ELISA assays.)
Benefits of an indirect Western
Indirect Western blots are very flexible. Labeled secondary antibodies are relatively inexpensive and high-quality, validated ones are easy to find. Antibodies that recognize all species of primary antibodies are widely available with a variety of different types of labels. Therefore, you can change-up the method of detection (say chemiluminescent versus fluorescent) by simply buying a new secondary antibody.
In addition, one secondary antibody can be used to recognize many different primary antibodies of the same species, leading to a cost savings.
If you listen to lab lore, you’ll hear that indirect Westerns are more sensitive than direct Westerns. This is based on the fact that multiple labeled secondary antibodies can recognize a single primary antibody, leading to an amplification of signal.
Disadvantages of an indirect Western
Before you begin your indirect Western, you should take the time to titrate both antibodies to get the maximum signal-to-noise ratio in your assay. This might take a bit longer than titrating only one antibody. The actual indirect assay also takes longer to do; you have an extra hour of incubation and about an extra half hour of washes, leading to more time on the bench.
Indirect Westerns can be “dirtier” than direct Westerns; the more antibodies you use in an assay, the higher the background. You might have to do some extra troubleshooting to determine which antibody is giving you the background.
Additionally, you might find the detection of multiple antigens simultaneously (multiplexing) using the indirect method a bit tricky. You have to make sure that your primary antibodies are from different species (or are distinctly different isotypes of the same antibody) so that the secondary antibodies don’t recognize the wrong primary antibody.
Direct detection
Direct detection is becoming more popular, particularly with the advent of fluorescent Westerns. In a direct detection Western, the primary antibody does the job of two antibodies: it binds to the protein of interest and it is directly labeled with an enzyme or dye for detection, shown in this picture:

Benefits of a direct Western
Direct Westerns are inherently simpler than indirect Westerns: they require less time, have a decreased number of steps/manipulations and are less prone to background.
Direct Westerns are particularly powerful when primary antibodies are labeled with fluorescent dyes. They can be used in highly quantitative assays.
Disadvantages of a direct Westerns
Direct Westerns were initially hampered by the chemistry involved in labeling antibodies. Chemical modification was mainly performed by specialty labs. However, labeling kits have been devised that enable labeling of antibodies in just about any laboratory setting. Now, you can label your antibody in under an hour IF you have a sufficient amount of relatively pure antibody.
However, you don’t want to label your primary antibody if you only have a little bit. The amount of antibody you have might be the deciding factor in the type of assay you do.
When using a labeled primary antibody, you might find that your assay isn’t as sensitive. You might have to load more protein or use more antibody to see the same level of signal. This is one of the factors that contributes to a cost increase with direct Westerns.
They can also cost more money if you don’t have an inexpensive or easily replenishable source of primary antibody (can you say hybridoma).
Finally, direct Westerns are not as flexible as indirect Westerns. You will have to label each and every primary you want to use.
As you can see, doing a direct or an indirect Western is not just a matter of deciding whether to use one or two antibodies. There are many factors to take into consideration when setting up your assay. Hopefully, we’ve just made the process a bit easier for you.
Photo courtesy of NIAID.

Leave a Reply