
So you have finally decided that you should do an ELISA instead of a Western blot. But your decision process isn’t quite done yet. Now you need to decide which type of ELISA to do. You could go the traditional route, or maybe it’s time for a sandwich*?
Let us help you decide.
Traditional ELISAs
When you perform a traditional ELISA, you first incubate the microtiter plate with your analyte. Your protein, along with all the other proteins in the sample, will bind to the plate. You then add antibodies that will recognize and bind to your target protein. It only involves a couple of steps and can be most likened to a Western blot.
This is shown in my kindergarten-like drawing below (seriously, my toddler could have done better). The circles represent various proteins in the sample with blue circles representing the protein of interest. The Y-shaped antibodies provide the specificity of the assay.

With a traditional ELISA, a mixture of proteins is bound to the microtiter plate. Your protein of interested is recognized by the antibody after binding to the plate.
Traditional ELISAs work well when your protein is abundant enough to give a strong signal despite the presence of irrelevant proteins competing for binding sites on the plate. Too many irrelevant proteins can decrease your signal-to-noise ratio. For this reason, traditional ELISAs are often the assay of choice when working with purified or partially-purified samples.
If using a traditional ELISA, you also need to make sure your antibody is truly specific for your target protein. If the antibody recognizes other proteins in the mixture, then your results won’t be accurate.
Sandwich ELISAs
When analyzing small amounts of protein within a complex mixture, most people turn to the sandwich ELISA.
In a sandwich ELISA, you first incubate the microtiter plate with an antibody specific for your protein of interest. This first antibody is called the “capture antibody.” You then add your analyte. The capture antibody will bind to your protein of interest and secure it to the plate while other proteins in the mixture are washed away.
The next steps are the same as a traditional ELISA. You incubate the plates with a second antibody (called the detecting antibody) that also recognizes your target protein. Thus, the antigen becomes “sandwiched” between two antibodies.
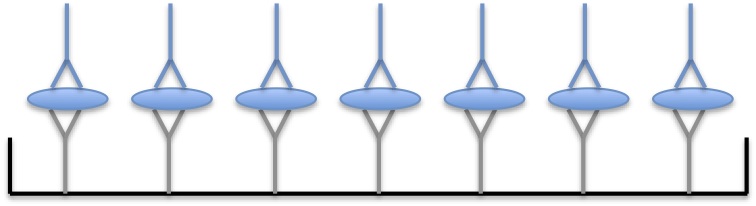
In a sandwich ELISA your protein of interest is captured to the plate using one antibody and then detected using a second antibody.
Sandwich ELISAs have several advantages over traditional ELISAs:
- They are 2-5 times more sensitive than traditional ELISAs
- You don’t have to purify your sample prior to analysis
- The specificity of the assay is increased because you are using two antibodies that recognize the same protein
- They can be used to study posttranslational modifications such as phosphorylation. e.g. the capture antibody could be directed against total protein while the detecting antibody only recognizes the phosphorylated form.
But sandwich ELISAs can be tricky. The target protein must have at least two distinct epitopes recognized by the two different antibodies. Matched antibody pairs used in sandwich ELISAs can be purchased from multiple companies.
A little note about nomenclature
Some people refer to traditional ELISAs as “direct” ELISAs because you detect your protein of interest directly without having to capture the antigen first.
But this should not be confused with direct detection vs indirect detection, two different methods of bridging the protein of interest of the substrate used for detection.
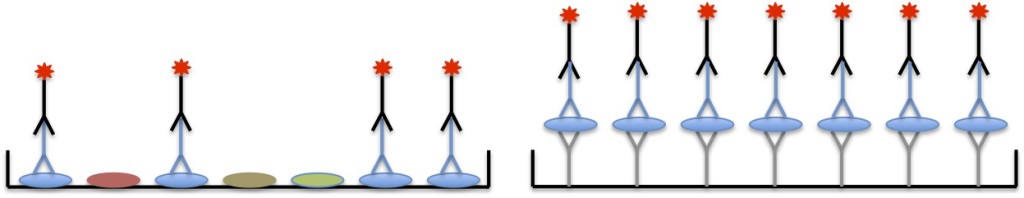
Direct detection
In direct detection, the primary antibody used to bind to the protein of interest is directly tagged with the substrate. This is shown in the illustration below, traditional ELISA on the left and sandwich ELISA on the right. For a traditional ELISA, only 1 antibody will be used throughout the entire assay while 2 antibodies will be used for a sandwich ELISA.
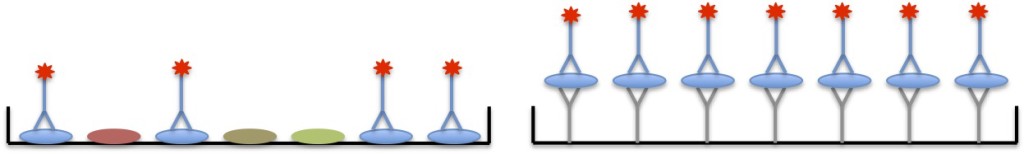
Indirect detection
Indirect detection is more popular than direct detection. Using the indirect method, you first incubate with a primary detecting antibody that recognizes the protein of interest, but is not bound to a substrate. You then use a labeled secondary antibody that recognizes the primary antibody. That makes for 2 antibodies in the traditional ELISA and 3 antibodies (whew!) in the sandwich ELISA, as shown below.
Warning: If you are using indirect detection with a sandwich ELISA, make sure the secondary antibody only recognizes the detection antibody and does not recognize the capture antibody, otherwise you will be in trouble. This can be accomplished by:
- Using capture and detection antibodies made in different species of animals
- Buying secondary antibodies that are highly cross-adsorbed against the host species of the capture antibody
*Disclaimer: We are not condoning eating in the lab. It’s dangerous, and well, just plain gross!
Photo courtesy of Susan Lucas Hoffman.

Leave a Reply