I love using fluorescent antibodies instead of the old-fashioned (well, I think they are) HRP-conjugated ones for Western Blots. If you work with dye-conjugated antibodies, you don’t stress over guessing the right exposure time, while your time’s ticking down. The signal from the fluorescent antibodies remains stable for much longer – days instead of minutes. You don’t have to squeeze into a dark room full of other stressed people. With fluorescent antibodies, you can detect more than one protein on one membrane. And lastly, greens and reds are lovely.
But I also find that using fluorescent secondary antibodies has significant disadvantages. Sometimes fluorescent secondary antibodies are much worse than HRP-conjugated secondaries. For example, they can react poorly with your primary antibody or non-specifically with your lysate. Maybe that’s why HRP-conjugated secondaries aren’t out of fashion yet. Also, in my experience, even when you’ve settled on a fluorescent secondary the quality varies batch-to-batch.
Wouldn’t it be great to retain all the benefits of fluorescence without the pitfalls? Directly labeling your primary antibodies with fluorescent dyes can be a simple path to creating beautiful fluorescent blots.
In what techniques can I use directly labeled primary antibodies?
Any technique that involves a fluorescent antibody. For example, flow cytometry, Western blotting, and immunofluorescence microscopy. You can use one antibody across multiple techniques, making the comparison easier. Using directly labeled primaries is extremely beneficial when multiplexing (using several antibodies in one experiment) with antibodies from the same species. This will quench the headache of sourcing secondaries from exotic species.
Isn’t my signal going to be weaker since I am not amplifying with a secondary?
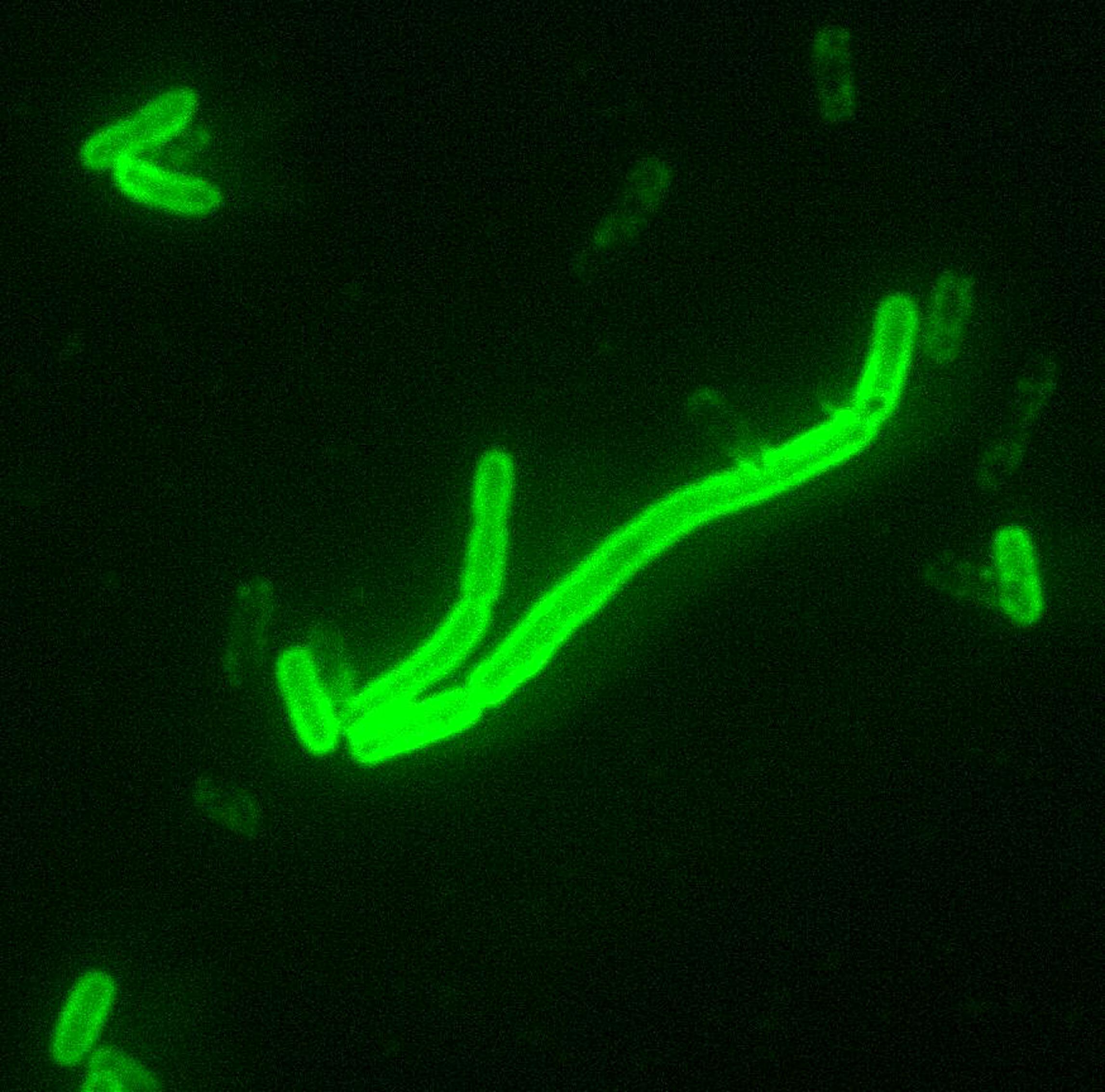
Yersinia pestis, Direct Fluorescent Antibody Stain. Photo courtesy of Center for Disease Control and Prevention (USA).
It may come as a surprise to you, but secondaries signal amplification is overrated. Because your primary doesn’t have an infinite affinity to the antigen, each time you wash your blot – and you wash a lot – you will remove some of your primaries. Using just primaries is usually enough for sufficient signal detection. As evidenced by those using peroxidase-anti-peroxidase antibodies (PAP) immune complexes, which don’t require secondaries.
Isn’t direct labeling complicated?
It used to be, but not anymore. The labeling reaction used to require biochemical techniques such as size exclusion chromatography to remove a free dye, but in modern times labeling is straightforward and simple.
For example, our Spectradye Antibody Labeling Kits allow you to label your antibody in as little as 30 minutes. And this 30 minutes will save you hours and hours of dealing with secondaries.
Doesn’t direct labeling require a lot of antibody?
Our SpectraDye Antibody Labeling Kits require as little as 10 μg of antibody. In this case, our trial kit is sufficient as you can label up to 100 μg of antibody. If you are pleased with your result, you can upgrade to our standard kit, which is sufficient for labeling up to 1 mg of antibody. Your antibody should be reasonably pure, though, at least, 95%.
Why use fluorescent dyes to label your antibodies?
A rule of thumb: if something is commercially available, it’s quick, easy and efficient relative to a home process. For example, modern cheap and cheerful DNA sequencing is possible because of kits containing fluorescently labeled dNTPs. Secondary antibodies with attached fluorescent dyes have a high signal-to-noise ratio increasing your sensitivity. They also have narrow emission spectra limiting signal overlap between the dyes.
With the recent advancements in imaging technology, new fluorescent dyes and the reduced cost of both, fluorescent detection systems are quickly gaining popularity. While the detection limits are still not as low as chemiluminescent detection, fluorescent detection has the unique advantage of allowing to assay multiple targets simultaneously on the same blot without the need to strip and re-probe.
Will SpectraDyes work with my current instrumentation?
We have 5 commonly used fluorescent dyes to choose from with emission range from 515 to 800 nanometers. But if you decide that you love your current dye too much to change, we also supply a kit without the dye so that you can use your own.
Let’s get labeling.

Leave a Reply