Revision for “Total Protein Normalization for Western Blots” created on November 2, 2015 @ 16:04:20
| Title | Total Protein Normalization for Western Blots |
|---|---|
| Content | <div class="level1">
Sample preparation, loading, uneven transfer and transfer efficiency can all affect recovery and detection of proteins on the membrane. When evaluating the amount of protein of interest on the blot, it is vital to use a normalization control. Traditionally, detection of a housekeeping gene is used for normalization to control for inconsistencies. However, housekeeping genes have specific disadvantages as normalization controls. Total protein normalization is another method for normalizing protein expression. Total protein normalization can be performed using different dyes and stains, as well as a newer stain-free technology. This guide reviews several different methods of total protein normalization.
</div>
<h2 id="housekeeping_genes_as_normalization_controls" class="sectionedit2">Housekeeping genes as normalization controls</h2>
<div class="level2">
To perform normalization using a housekeeping gene, the blot is probed with antibodies to detect the protein of interest while another set of antibodies is used to detect a separate protein used as a normalization control. For quantitative studies, the ratio of the abundance of the protein of interest to the normalization control is used to quantify the amount of the protein of interest in each sample. For qualitative studies, the loading control is often presented in an image for visual comparison.
Housekeeping genes, such as such as glyceraldehyde 3-phosphate dehydrogenase (GADPH), beta-actin or tubulin, are commonly used for loading controls. These proteins are usually expressed constitutively at high levels due to their role in cell viability. However, <a href="https://advansta.com/housekeeping-genes-western-blots/" target="_blank">several factors limit their utility as normalization controls</a>.
<ul>
<li class="level1">
<div class="li">Studies have shown that housekeeping gene expression can change with different experimental conditions and differ between cell types and during different developmental phases (Liu, 2006; Moskowitz, 1995; Nahlik, 2003).</div></li>
<li class="level1">
<div class="li">The high level of expression of housekeeping genes often makes it difficult to detect a lower expressing protein on the same blot. The amount of protein required per lane to detect the protein of interest often results in a signal that far exceeds the linear dynamic range when detecting a housekeeping gene.</div></li>
<li class="level1">
<div class="li">For true normalization, the protein of interest and the normalization control need to be detected on the same blot. Unless the two proteins are sufficiently separated by gel electrophoresis and the membrane can be cut and incubated for each protein separately, many researchers rely on<span class="Apple-converted-space"> </span><a class="urlextern" title="http://www.advansta.com/blog/stripping-doesnt-pay-western-blots/" href="http://www.advansta.com/blog/stripping-doesnt-pay-western-blots/" rel="nofollow">stripping and reprobing</a>, which is not quantitative.</div></li>
<li class="level1">
<div class="li">Expression of the protein of interest is only compared to one other protein.</div></li>
</ul>
</div>
<h2 id="total_protein_normalization" class="sectionedit3">Total Protein Normalization</h2>
<div class="level2">
<a href="https://advansta.com/total-protein-normalization-western-blots/" target="_blank">Total protein normalization (TPN)</a> is a technique that can be used to quantify the abundance of the protein of interest without relying on housekeeping genes. Traditionally, TPN is performed by incubating the membrane with a total protein stain, either before or after detection with antibodies. The abundance of the protein of interest is normalized to the total amount of protein in each lane, removing variations associated with comparing abundance to a single protein. TPN is also more compatible with detecting proteins of lower abundance.
Normalization with total protein stains can be complex and time-consuming. While some stains can be used prior to immunodetection, others are incompatible with downstream antibody detection. In addition, some stains fade quickly making it difficult to document the results. A newer technique, called the stain-free approach, increases the sensitivity and reduces the complexity while saving time.
</div>
<h3 id="total_protein_stains" class="sectionedit4">Total protein stains</h3>
<div class="level3">
Total protein stains bind to all proteins on a Western blot membrane and provide a visual image after transfer. Depending on the type of stain, total protein stains can be used either prior to or after immunodetection. Some of the more common stains are described below.
</div>
<h4 id="pre-antibody_stains">Pre-Antibody Stains</h4>
<div class="level4">
Anionic dyes, such as<span class="Apple-converted-space"> </span><a class="urlextern" title="http://advansta.com/AdvanStain_Ponceau/?root=356/" href="https://advansta.com/products/AdvanStain_Ponceau/" target="_blank" rel="nofollow">Ponceau S</a>, and fluorescent dyes, like Sypro Ruby, Deep Purple and<span class="Apple-converted-space"> </span><a class="urlextern" title="http://advansta.com/AdvanStain_Scarlet/?root=356/" href="https://advansta.com/products/AdvanStain_Scarlet/" target="_blank" rel="nofollow">AdvanStain Scarlet</a><span class="Apple-converted-space"> </span>are common stains used prior to antibody staining.
</div>
<h5 id="ponceau_s">Ponceau S</h5>
<div class="level5">
Ponceau S is rapid and economical. Proteins stain red after just 5 minutes of incubation. The dye is easily removed after visualization by incubation in PBS or wash buffer. Although Ponceau S has no effect on downstream immunodetection, the intensity of the staining decreases quickly over time, making it difficult to capture an image. The red bands can also be difficult to photograph.
</div>
<h5 id="fluorescent_stains">Fluorescent stains</h5>
<div class="level5">
Although more expensive and time-consuming than Ponceau S, fluorescent stains are highly sensitive, permanent, total protein stains that can also be used prior to antibody detection. Several companies sell fluorescent stains that have limits of detection ranging from 1.0 ng-8.0 ng. The stains are long-lasting and photostable, enabling long exposure times. Most stains can be excited with either UV or visible light and require a fluorescent imager for detection. Staining can be documented on photographic film or with a CCD camera. The staining procedure can take 30 minutes – 1 hours, depending on the stain.
Note: Some stains contain heavy metals, which require special disposal procedures.
</div>
<h4 id="post-antibody_stain">Post-Antibody Stain</h4>
<h5 id="amido_black">Amido black</h5>
<div class="level5">
Amido black is a commonly used permanent post-antibody anionic stain. Although not as sensitive as a fluorescent stain, it is more sensitive than Ponceau S and does not require special equipment for visualization. It is also more economical than fluorescent stains. The bright black bands are easy to document.
</div>
<h5 id="colloidal_gold">Colloidal Gold</h5>
<div class="level5">
Colloidal gold particles can be used as a total protein stain. When incubated with proteins bound to a membrane, the gold adsorbs to the proteins through electrostatic interactions resulting in a transient, reddish-pink color. The sensitivity can be enhanced through sliver enhancement, which results in a stable dark brown signal and detection of protein down to 1 ng. Colloidal gold staining is more expensive than other methods and cannot be used with downstream immunodetection.
</div>
<h3 id="stain-free" class="sectionedit5">Stain-free</h3>
<div class="level3">
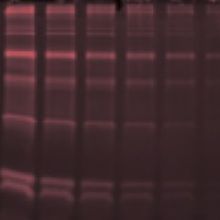
Stain-free total protein detection is rapid and sensitive. The technology uses a trihalo compound that is directly incorporated into the gel. Upon UV exposure, the compound modifies tryptophan residues in proteins causing them to fluoresce. The fluorescent signal can be detected by a CCD camera. Proteins can be visualized in the gel prior to transfer and after transfer on the membrane. Stain-free detection does not interfere with downstream immunodetection. Pre-cast gels stain-free gels can be purchased or stain free gels can be made in the lab by mixing a trihalo compound with acrylamide. Stain-free technology cannot detect proteins that do not contain tryptophans and it is recommended that a protein contain at least 2 tryptophans to be readily detected.
<a class="media" title="tpn_table.jpg" href="http://www.advansta.com/wiki/lib/exe/detail.php?id=total_protein_normalization&media=tpn_table.jpg"><img class="mediacenter" src="http://www.advansta.com/wiki/lib/exe/fetch.php?media=tpn_table.jpg" alt="" /></a>
</div>
<h2 id="tips_for_total_protein_normalization" class="sectionedit6">Tips for Total Protein Normalization</h2>
<div class="level2">
<ol>
<li class="level1">
<div class="li">Avoid edge effects. Dyes and stains can stain the edges of the blot more darkly than the interior of the blot.</div></li>
<li class="level1">
<div class="li">Load lanes in duplicate or triplicate randomly across the gel</div></li>
<li class="level1">
<div class="li">Use enough stain/dye to completely submerse the blot</div></li>
<li class="level1">
<div class="li">Shake blot while staining/destaining blot to distribute stain/dye evenly</div></li>
<li class="level1">
<div class="li">Be sure gels are loaded evenly.</div></li>
<li class="level1">
<div class="li">Mix samples thoroughly and spin contents to bottom of tube before loading</div></li>
<li class="level1">
<div class="li">Establish range of linearity for each dye or stain.</div></li>
<li class="level1">
<div class="li">Run a dilution series on a gel and stain to determine the range of protein that can be detected linearly</div></li>
</ol>
</div>
<h3 id="references" class="sectionedit7">References</h3>
<div class="level3">
Liu NK, Xu XM. beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. J Neurotrauma. 2006;23:1794–801.<span class="Apple-converted-space"> </span><a class="urlextern" title="http://www.ncbi.nlm.nih.gov/pubmed/17184189/" href="http://www.ncbi.nlm.nih.gov/pubmed/17184189/" rel="nofollow">PubMed</a>
Moskowitz PF, Oblinger MM. Transcriptional and post-transcriptional mechanisms regulating neurofilament and tubulin gene expression during normal development of the rat brain. Brain Res Mol Brain Res. 1995;30:211–22.<span class="Apple-converted-space"> </span><a class="urlextern" title="http://www.ncbi.nlm.nih.gov/pubmed/7637572/" href="http://www.ncbi.nlm.nih.gov/pubmed/7637572/" rel="nofollow">PubMed</a>
Nahlik KW, Mleczko AK, Gawlik MK, Rokita HB. Modulation of GAPDH expression and cellular localization after vaccinia virus infection of human adherent monocytes. Acta Biochimica Pol. 2003;50:667–76.<span class="Apple-converted-space"> </span><a class="urlextern" title="http://www.ncbi.nlm.nih.gov/pubmed/?term=nahlik+mleczko/" href="http://www.ncbi.nlm.nih.gov/pubmed/?term=nahlik+mleczko/" rel="nofollow">PubMed</a>
</div> |
| Excerpt |